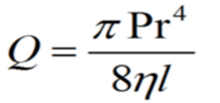Original Article 1, Issue 11.3
A Novel Method for Pre-selectivity of Oral Appliance Palliative Pharyngeal Airway Outcomes in Obstructive Sleep Apnea: A Pilot Study
http://dx.doi.org/10.15331/jdsm.7342Bruce S. Haskell, DMD, PhD1,2, David Jensen, DMD, MS3, Andrew M. Roberts, PhD2 †, Mohamed Bazina, DMD, MSD1
1University of Kentucky Department of Orthodontics, 2University of Louisville Department of Physiology, 3University of Florida Department of Orthodontics
ABSTRACT
Objectives:
A qualified clinical solution improves preselective probabilities regarding who may successfully use an oral appliance (OA) for obstructive sleep apnea (OSA) for upright-awake oropharyngeal airway axial cross-sectional improvement. A discrete value method was constructed, measuring morphologic typology cone-beam computed tomography elements for OA use.
Materials and Methods:
Patients (n=20) in whom OSA was diagnosed and for whom OAs were prescribed were randomly selected for upright wakefulness airway imagery based on limited availability of imaging with and without OA placement. Cross-sectional axial airway areas were calculated and divided into “good” and “poor” responders (≥16% or <16%, respectively) for airway change. An OA evaluation index, using discrete scoring methods based on morphologic typology, was constructed to evaluate the effectiveness of OA usage for upright awake minimal axial airway dimension improvement, providing a predictive model for anatomic responder type.
Results:
Using the oral appliance efficacy index, “good” and “poor” responders for upright awake axial airway area increase was predicted at a 70% accuracy (P=.02) from derived two-dimensional cone-beam computed tomography cephalometric values without the inclusion of the middle pharyngeal muscle vector change and 75.5% (P=.05) when middle pharyngeal measures were included.
Conclusion:
Discrete scoring using cephalometric measures and middle pharyngeal muscle vector length change predicted OA palliative pharyngeal airway change. A 75.5% predictability for upright awake patients with OSA achieving a minimal cross-sectional axial airway area increase greater than or equal to 16% using an OA device was found, for a calculated 77.5% increase of airway flow.
Clinical Implications:
Who may increase airway dimensions is an effort, money, and time saver.
Keywords:
four-bar analyses, obstructive sleep apnea, oral appliance (MAD), oro-pharyngeal predictability
Citation:
Haskell BS, Jensen D, Roberts AM, Bazinga M. A novel method for pre-selectivity of oral appliance palliative pharyngeal airway outcomes in obstructive sleep apnea: A pilot study. J Dent Sleep Med. 2024;11(3)
INTRODUCTION
Examination of morphologic typologies and biomechanical factors presents a method to improve reliability of oral appliance (OA) use by preselecting upright-awake good versus poor responders to minimal axial airway area increase in patients with obstructive sleep apnea (OSA).
OSA occurs when airflow is obstructed by anatomic structures during sleep. This decrease in oxygen to the lungs is associated with hypopnic disturbances as sudden interruptions to sleep. Common risk factors for OSA include male sex, neck size larger than 17 inches, obesity, aging with oropharyngeal flaccidity, and snoring. OSA outcomes include daytime fatigue, cardiovascular events, high blood pressure, myocardial infarction, and stroke.1 Despite the severity of comorbidities, fewer than half of all patients with OSA undergo treatment.2 Treatment begins by obtaining a polysomnogram, and waking events are divided by sleep hours to calculate an apnea-hypopnea index (AHI). AHI higher than 5 events/h and less than 15 events/h is categorized as mild; a score of 15 to 30 is considered moderate, and a score higher than 30 is noted as severe. The standard of care is continuous positive airway pressure or bilevel positive airway pressure.3 Other treatment options include surgical advancement of the jaw(s), which allows anterior positioning of airway structures, increased airway volume, and alternatively, stimulation of the hypoglossal nerve.4 Palliative function of OAs for OSA is to reduce apneic episodes through improvement in airway patency. Positioning the mandible forward and opening the airway is intended to increase airway volume and resolve possible constriction points.5
Limited data are available on how OAs may interactively influence physiologic variables. For example, the hypoglossal nerve enables control of the hyoglossus, intrinsic, genioglossus, and styloglossus muscles. Advancing the tongue with an OA, these muscles help open the airway. Hyoid position with an OA is also altered, and together with the tongue and mandibular position helps to regulate pharyngeal airway dimensions. Forward positioning of the mandible with an OA can increase velopharyngeal and genioglossal tension, opening the pharynx an unspecified amount, while the genioglossus and geniohyoid musculature hold the tongue forward and anteriorly from the back of the pharynx, preventing airway occlusion. Advancement with an OA reportedly increases basal electromyographic activity of the genioglossus, activating the palatoglossus and palatopharyngeal muscles and lateral walls of the velopharynx with an increased vertical dimension. This indirectly affects tonus of lateral wall tissue. Airway baroreceptor/pressure receptors maintain systemic blood pressure with changes in orientation.6.
Older age, body mass index (BMI), neck size, hypoglossal nerve function, and neuromuscular feedback, including lateral wall function, all are physiologic multifactorial elements associated with OSA. Demographic factors such as younger age, female sex, lower BMI, smaller neck circumference, retracted maxilla and mandible, narrower airway, shorter soft palate, and lower OSA severity are phenotypic features of good responses to OA use. 5,6
OAs are prescribed by physicians as recommended by the American Academy of Sleep Medicine to patients with an AHI less than 30 events/h. 5
Limited Usefulness of OAs
Prior studies show limited correlations between OAs and improved AHI responses with standard deviation (SD) values of reportedly successful treatment often approaching or exceeding the mean. A scoping review indicated individual OA application unpredictability with wide variability of AHI, with 61.81% ± 12.29 related to predisposing factors.5 One study reported only 39% of all patients may respond clinically with an OA advancement.6
Studies report weak anatomic significance, with only general trends identifying no universal tendencies related to OA response. Negative OA outcomes may include unpredictable airway shape and volume changes, minimal axial airway areas, vertical position changes of the narrowest airway constriction point, and canted hyoid positions and angulations as well as limiting the extent of anterior mandibular positioning through mandibular autorotation. 5-17
OA prescription measures appear inconsistent and often indeterminate for palliative treatment of OSA. This lack of correlation may be based on unknown or incorrect assumptions of airway anatomy and kinematic effects. 5,18,19 The study authors showed that AHI/sleep disturbed breathing related to a specific anatomy is inconclusive. 5 Measuring individual anatomic structures or using jaw advancement alone to treat patients with OSA instead of an interaction of associated components appears simplistic.20,21
A Topologic Focus for Airway Predictability
This pilot presents an anatomic-typologic approach to airway volume improvement for patients in whom OSA is diagnosed as a partial answer to OA preselectivity. A correlative conclusion relating sleep studies to verify the increase in airway as resolving sleep disturbed breathing is not intended. Instead, selected craniofacial typologies reveal the potential for improvement in pharyngeal minimal axial airway in upright awake patients by identifying anatomic and biophysical changes with an OA. It is intended as a qualified practical preselective anatomic assessment in the recognition of “good” from “poor” responders for improvement in airway components as who may successfully use an OA for an enhancement of upright awake minimal axial airway dimensions.
MATERIALS AND METHODS
Patients treated for OSA at the University of Louisville (n=20) self-reporting a subjective improved sleep response to OSA therapy with an OA were randomly selected for Herbst-type OAs from a very limited existing patient population based on availability of cone-beam computed tomography (CBCT) with and without OAs. Patients obtained from dental radiology archives were blinded from demographic and other physiologic data. Pretreatment and posttreatment objective sleep PSG data (including AHI) were not available for the cohort of patients. No additional sample populations diagnosed with OSA available with and without an OA with CBCT imagery were discovered. For diagnosis and treatment, two CBCT images (i-CAT, Norcross GA) were obtained with the patient awake in an upright sitting position: one image showing maximum intercuspation and the second with a Herbst-style OA positioning the jaw 75% of maximum protrusion. Because sleep imaging units are not employed in ordinary clinical practice, this retrospective study used available CBCT, employing a standard upright awake unit. Boundaries of each scan were full cranial volumes extending to below the hyoid, except for one patient with a scan located by the superior boundary at the Frankfort horizontal plane. Data collection was approved by an institutional review board at the University of Florida College of Dentistry (IRB # 202.06).
Imaging and Measures
Scans were deidentified and imported as DICOM files into Dolphin 3D Imaging Software (version 11.95, Chatsworth, California) via a secure server to the University of Louisville under project approval of IRB202002352, with AHI values blinded from examiners. The airway analysis tool within Dolphin Imaging was used to define the sagittal borders of the airway by a box with vertices at the posterior nasal spine, basion, the anterior inferior border of the C3 vertebrae, and the central body of the hyoid. The area of interest was defined by a “seed” placed in the airway space toggled to ensure a smooth border of the lumen. The analysis tool calculated total volume and minimum axial airway areas for each patient (Figure 1). The difference in the length of the vector of the middle pharyngeal constrictor muscle was obtained from both appliance and nonappliance CBCT images. Vector length was measured from the pharyngeal tubercle to the greater horn of the hyoid. Tracings were completed using the “Digitize” tool on all extracted cephalograms in maximum intercuspation by using the “Build X-ray” tool from each CBCT image.
Three-dimensional superimpositions of each patient with and without the OA in place were performed with airway changes calculated from the difference in measures with and without appliances in place.
These measures are associated with mandible/airway position: ANB, (skeletal classification) WITS (anteroposterior jaw position): the position of the maxilla and mandible relative to each other and their relation to the occlusal plane. Overbite depth indicator (ODI): the plane connecting A point from B point to the mandibular plane angle plus or minus the palatal plane to Frankfurt horizontal plane, and the middle pharyngeal constrictor muscle vector length5,22 (Appendix 1). Airway volume and upright awake minimal axial areas were measured, made from the calculated lateral cephalometric (two-dimensional) image from CBCT.
A disadvantage of practitioner accessibility using in-office CBCT is the need for upright awake posture during imaging instead of using a sleep functional CBCT/MRI. The authors thought an average airway measure with CBCT was perhaps a best compromise for assessment of axial volume. CT performed in the hospital with the patient supine is a source of high millisieverts and not available.
Dimensional Aspects of Airflow
The narrowest axial lumen follows the Bernoulli principles of a narrowed aperture: increased velocity, more turbulence, and reduced volume of flow. The responders were divided into respective subgroups, expecting that the collected data would follow a standard distribution. To divide “good” from “poor” enhanced anatomic responders, a threshold value of 1 SD below the mean was selected. A threshold value of -1 SD translated to an equal value of a standard score. When applied to a negative value z-table it yields a value of 0.15866 or 15.866%, and when rounded to the nearest whole number is 16%. Thus, a 16% upright awake minimal axial airway increase was used as the division between “good” and “poor” enhanced anatomic responders. This is judged substantial as the Hagen-Poiseuille laminar flow equation conveys exponential output of flow23 (Q = flow, P = pressure, r = tube radii, l = length of tube, n= viscosity of air). If all variables of the equation are standardized between two events and the only variable to change was the radius, then given an event with a radius =1, the flow (Q) would be 0.4. If the radius is increased by 16% (r =1.16) the output of this model is Q = 0.71. This yields a 77.5 % increase of flow: a reasonable threshold for dividing responder types. The radius of the pharynx increases with advanced jaw position; the area from transverse and anteroposterior movements decreases airway resistance by a factor of the radius to the fourth power.7,23
Anatomically, good responders higher than a 16% threshold have substantially enhanced upright awake minimal axial dimensions, whereas poor upright awake axial airway enlargement responders to a minimal extent below a 16% threshold may also experience some improved airflow based on the laminar flow equation.
Airway volume and minimal axial areas were measured in all upright awake patients. Intraevaluator reliability evaluation was completed by a selection of three cephalometric variables measured twice, 1 month apart, on five different cephalometric radiographs. The kappa score was calculated (0.89) for reproducibility and consistency. Quantitative and qualitative observations were recorded with respect to modeling the highest values for both specificity and sensitivity.
RESULTS
A change in cross-sectional airway area occurred with all 20 upright awake patients in the pool for both good and poor responders. Overall, this represented an average improved axial airway area of 27% with an OA. Eleven individuals were identified with an increased upright awake minimal axial area greater than or equal to 16% (good responders) and 9 individuals with an axial area less than 16% (poor responders) (Table 1). The good responders presented an average 56.6% minimum upright awake axial airway area improvement, while the poor responders averaged a decrease of 2.5% upright awake axial airway area change.
The Oral Appliance Efficacy Index
The oral appliance efficacy index (OAEI) is a constructed discrete variable classification index of selected morphologic variables using weighted scores to predict the axial cross-section of the airway with an OA at the threshold of 16%. Datasets are considered discrete if the values belonging to the set are distinct and separate; similar methodology is applied in the Pediatric Sleep and STOP-BANG disturbed sleep questionnaires.24,25 Reviews indicated that continuous variables of cephalometric data are limited for predicting airway.5 Here, specific typology cephalometric value composite scores of discrete measures were developed to evaluate OA predictive improvement for minimal axial airway area change in upright awake patients.
The OAEI allows prediction of good or poor upright awake minimal axial airway response based on a numerical threshold. Table 2 shows the four variables that were considered: ANB, WITS, ODI, and the change of the length of the middle pharyngeal muscle vector when an OA is placed. If a patient met specific threshold values, they were given the corresponding point values: ANB greater than 4, WITS greater than 3, ODI greater than 70, middle pharyngeal muscle change greater than or equal to 3. The point values were ascribed based on the SD of the normative values for each variable, with the greater the SD corresponding to a larger assigned point value. OAEI weights were based on required measures with two of the scores selected as 1 SD from the norms. The WITS score required an OAEI of 3 SD from the norm because a WITS SD is small. A WITS higher than 3 SD embodies mandibular retrusion. Because there is no current mean value for the length of the middle pharyngeal muscle length, the median score was assigned as an OAEI point value. The sum of these values ranging from 0 to 8 becomes the calculated score (Appendices 1, 2, and 3).
To prevent self-confirmational bias of the data, a random selection of half of the patient pool was used to identify the proposed variables before the complete cohort would be tested against the extrapolated threshold values. All patients (11 good and 9 poor anatomic responders) were assessed with the OAEI twice, once with ANB, WITS ODI, and the change of the middle pharyngeal muscle length after placement of an OA, and again using only ANB, WITS, and ODI scores.
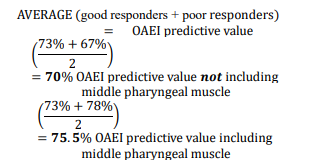
OAEI without inclusion of the middle pharyngeal muscle vector length change (Table 3): Threshold of response type was set with an OAEI score of greater than or equal to 3. Of the 20 patients evaluated, 8 of the 11 good responders (73%) met the expected outcome, whereas 6 out of 9 of the poor responders (67%) met their expected outcome. Pooling these data, the overall predicted outcome for evaluating the efficacy of the OAEI on the test patients in this dataset was 70%, with a value of P = 0.02.
OAEI including vector length of middle pharyngeal muscle change (with and without the OA) (Table 4): The threshold including the middle pharyngeal muscle vector length change was set at an OAEI score of greater than or equal to 5. The OAEI value threshold was increased in this iteration of the index because of the inclusion of an additional variable (the middle pharyngeal muscle length change). When applied to the 20 patients in this study there was no difference with the index not including the middle pharyngeal muscle, because 8 of the 11 good responders met the expected outcome with an accuracy also equaling 73%. However, the accuracy increased to 78% when evaluating poor responders, with 7 of 9 patients meeting the expected lowered criteria. Together, the combined predictive value for good and poor anatomic responders was 75.5% (P = 0.05), calculated by the average of accuracy in both responder types being correctly identified.
Figure 1Airway capture and constructed borders. CBCT images display a green box with vertices at the following points: posterior nasal spine, basion, the anterior-inferior border of the third cervical vertebral body, and the central body of the hyoid bone. Sagittal perspective of airway borders and seed point (yellow dot) to define airway rendering. |
Figure 2The interactive nature of airway patency. At least 36 possible interactions are noted for muscle vectors, skeletal anatomy, jaw position, and airway lumen. |
Figure 3A distribution of patients according to OAEI score. “Good” anatomic responders (blue) are located at the highest scoring end of the distribution with two outliers that scored zero points. “Poor” anatomic responders (red) have lower scores on average; being found at the lower end of the score table. |
Figure 4Examples of outlier and non-outlier good responders A, Good responder outlier with a poor OAEI (Class III tendency). B, Good responder with a good OAEI (Class II tendency) The airway appears small with Class II characteristics (B). Dimensions of the oropharynx may be larger in prognathic types (A). |
Figure 5Four-bar analysis with and without OA in place. A four-bar closed chain linkage with bars and four joints with three degrees of freedom using straight-line two-dimensional vectors moving in parallel. A, Without OA. Geniohyoid muscle (red): mandibular genial tubercles to hyoid. Hyoid bone (light blue). The middle pharyngeal muscle vector (yellow) from the pharyngeal tubercle of occipital bone to anterior of styloid process / posterior portion of hyoid. Mandible (dark blue) to pharyngeal tubercle. The links move in parallel. B, With OA. C, CBCT image section of middle pharyngeal constrictor vector (yellow). |
Tables 1 and 2 |
Table 3 |
Table 4 |
DISCUSSION
Stomatognathic System
Although ideal for evaluating the airway of patients with OSA, sleep functional CBCT/MRI performed in the supine position is not typically available in an office setting. With practitioner-accessible in-office upright CBCT, an awake posture is lamentably requisite. Methodology using CBCT images obtained from upright awake patients with OSA for improving the probabilities for OA efficacy is understood as qualified. It may be considered a practitioner based, improved method over indeterminate OA construction with limited effectiveness. Preselective enhanced methodology CBCT measures of OA usage for airway improvement may support or eventually circumvent multidisciplinary invasive methods such as drug sleep-induced endoscopy to determine whether optimal jaw positioning is acceptable for patients with OSA.26
Idiosyncrasies of Mandibular Advancement for Airway Enhancement
Specific mechanisms for developing airway patency with OAs are not well understood. A conundrum exists in that all patients subjectively self-reported “improvement in sleep”, whereas the data indicate poor anatomic responders did not experience an improvement in upright awake axial airway volume. A recent paper using OAs (mandibular advancement device [MAD]), measuring AHI improvement reports, partly clarifies this enigma with similar findings. “At first follow-up after MAD delivery, non-responders (no AHI improvement) reported less tiredness upon awakening (P = 0.003), better sleep quality (P = 0.005), and greater subjective improvement (P = 0.012) than (AHI improvement) responders. Among significant OSA symptoms, tiredness upon awakening, poorer sleep quality, and less subjective improvement were consistently found as predictors of (AHI improvement) treatment response…. This incongruity further complicates the determination of an appropriate endpoint to MAD advancement by the qualified dentist as in routine clinical setting…”6
Enigmatically, upright awake poor anatomic responders as less than 16% axial airway enlargement, subjectively self-reporting sleep improvement, is also reported. A small portion of the poor responder patients may have some degree of patency improvement due to mild airway improvement and unknown factors possibly indirectly affecting lateral wall tissue tonus.6 Additionally, a placebo effect may exist known psychologically as response bias / courtesy bias, or placebo effect. With the placebo effect, a patient with an OA wants to assist in the treatment process by telling the practitioner what he desires to hear. Ideally, all subjective patient responses should be properly verified by measurable data as with polysomnography.
Differences in mandibular motion and structure relate to unpredictable alterations of airway volume.27 Appliances protruding the mandible often combine with confounding vertical anatomic and rotational effects. For example, patients with hyper-divergence and increased clockwise rotation of the mandible on wide opening can reposition soft and hard tissues, moving the tongue posteriorly and obstructing airway structures.5 Compounding this effect, obtusely angled OA distention jigs and appliance thickness may produce unwanted vertical effects due to disocclusion before protrusion. Increasing vertical elements lead to mandibular posterior rotations causing a 0.3-mm reduction in the range of mandibular advancement for every 1 mm of vertical opening.6 For patients with a backward opening pattern of Posselt envelope of motion, an OA may be even less effective for OSA treatment.5-13 Only 50% to 61% AHI reduction is typically gained using OAs for OSA.5,19,20 Application of OAs is often indiscriminate, relying on idiosyncratic aspects of muscular tension of pharyngeal musculature to improve airway instead of nonmuscular inflation with a continuous positive airway pressure/bilevel positive airway pressure for patency.5 It is beyond the scope of this structural model to incorporate additional variables of neuromuscular, central nervous system, or oro-pharyngeal flaccidity, obesity, and aging issues. Instead, 36 different possible interactive anatomic aspects of OSA are suggested28 (Figure 2). Certain associations have previously been identified as balancing factors contributing to interconnectivity for patency, including: airway lumen, genioglossus electromyogram activity, biomechanical influence, and pharyngeal shape.5 A relationship between AHI reduction with a specified minimal axial airway area with OA use is plausibly implied and requires further study.
Classification of group typologies
Mandibular retrusion with a deficient oropharyngeal area is a known predictor for AHI reduction when using an OA. This typology includes vertical facial types, including those with deficient ramal height, open bite tendency, and an inferiorly placed posterior nasal spine.5,7,9,12 No current method is ideal because of multifactorial etiologic inputs on the presentation of OSA. This is represented by the variance shown in Figure 3. If OAEI presented a perfect predictive measure, there would be two distinct peaks in this figure; however, employed as a qualified diagnostic tool, outliers are not unexpected. Patients with a good anatomic response and with low OAEI scores considered outliers are explained by myriad factors not fitting the proposed model. For example, patients with Class III prognathism often display enlarged pharyngeal airways without mandibular retrusive characteristics and yet respond well to OA 29 (Figure 4). Specificity issues may include but are not limited to age, poor neuromuscular compensation (electromyography), BMI, neck circumference, airway shape and hyoid position.
Four-Bar Biomechanical Analysis
Alteration of the middle pharyngeal constrictor is associated with changes in airway- generating potential. This pharyngeal muscle allows the oropharynx to open efficiently with advancement of the mandible. Electromyography of muscle function requires invasive procedures whereas MRI for three-dimensional muscle origin and insertion were unavailable.
Instead, a four-bar analysis is used, which consist of links that move relative to one another, measuring both displacement and angular change.30 The four-bar graphic in Figure 5 is used to assess altered elements of change with angular jaw and hyoid movements limited to a two-dimensional vector estimation of a complex three-dimensional structure. The middle pharyngeal muscle vector approximates both convoluted space and shape.
OA palliative alteration of the of the middle pharyngeal muscle is related to reports that dilator muscle tone may alter airway.31,32 This constrictor (yellow bar) can change vector angulation with appliance use, and may lengthen, shorten, or remain unaltered with OA placement. Jaw advancement with lengthening of this muscle may plausibly induce extra play or creep into the four-bar system. This suggests the middle pharyngeal musculature/hyoid link can function as a slider-crank linkage with a mild lengthening trend in those with less response to an OA. Including the muscle vector change increases the specificity of the OAEI from 70% to 75.5%.
The discrete variable scoring was established with and without this measure using percentages of predictive reliability because not all clinicians have access to CBCT, whereas others may prefer not to deliver additional radiologic exposure.
Clinical Worksheet
A worksheet (Appendices 1, 2, and 3) is provided using cephalometric weighted variables (OAEI ≥3) for clinicians to improve airway prediction percentages for patients who may or may not benefit from a palliative OA. Only one cephalometric image is required for predicted accuracy of 70%. If an increase of predicted accuracy to 75.5% is desired, CBCT should be performed twice: first in centric relation and second with a wax bite simulating an activated OA with three-fourths protrusion. The difference in the images of the middle pharyngeal muscle vector length from the pharyngeal tubercle to the hyoid is calculated for a complete score (OAEI ≥5) evaluation. The pharyngeal tubercle lies on the lower surface of the basilar portion of occipital bone and is the attachment of the pharyngeal raphe.
The raphe is the insertion for the pharyngeal constrictors including the middle pharyngeal constrictor. It originates from the greater and lesser cornu of the hyoid, inserting into the raphe. The yellow arrow represents a simple vector used for the length of this muscle in the OAEI supplementary analysis.33 For imaging of the pharyngeal tubercle for the four-bar analysis, CBCT is suggested to facilitate the location of this structure. Although it is possible to visualize an estimation of pharyngeal tubercle position with a lateral cephalometric image, CBCT ensures accuracy of imaging of the tubercle structure itself. Many practitioners now eschew the cephalometric x-ray machine and use a composite image of all dentition, cephalometric image, airway, adenoids, and tonsils, etc. derived from CBCT alone (Appendices, Figure 5).
Limitations of this study
Placement of an OA device for simple mandibular advancement to ameliorate OSA without a planned outcome is an expensive invitation for limited effectiveness or outright failure.34 The application of composite biometric typology in a limited pilot study of 20 upright-awake patients for an anatomic-specific CBCT analysis without polysomnogram data (PSG), BMI, family history, and other pertinent considerations is conceived as preliminary groundwork for future OSA studies. Incorporating muscular bioengineering analysis and polysomnographic, demographic, and physiologic data, as well as validating subjective curative claims, is considered essential for improving predictive accuracy for successful application of OA devices. Prospective studies to validate a correlative key OAEI scoring for AHI reduction using demographics of age/sex/ BMI/ neck circumference/ ethnicity, etc. together with polysomnography and sleep functional CBCT / MRI are central. Larger datasets are required to resolve whether the pilot- constructed OAEI is validated for use as a reliable predictor of increased upright-awake minimal axial airway area with an OA, and whether the upright-awake CBCT axial area airway percentage improvements for “good” anatomic responders match patients with OSA treated with OA for improved PSG reduction scores.
CONCLUSIONS AND RECOMMENDATIONS
* The pilot OAEI model is a constructed, weighted index identifying typologies allowing a simplified quantification for preselective predictability of an OA for upright awake oro-pharyngeal airway enhancement.
* Using CBCT cephalometric measures, the model predicts upright awake airway enhancement for an OA in 70% of patients with mild to moderate OSA. Including the middle pharyngeal constrictor muscle with these data is proposed to predict upright awake airway enhancement of an OA in 75.5% of patients.
* Good anatomic responder values existing at a threshold of greater than or equal to a 16% upright awake minimal axial airway area yield an increased flow rate of more than 77.5%.
ACKNOWLEDGEMENT
The authors thank Dr. WC Scarfe, Chair, Department of Diagnosis and Oral Health. University of Louisville School of Dentistry for access to records.
REFERENCES
- Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291(16):2013–2016. doi:10.1001/jama.291.16.2013
- Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir Med. 2019;7(8):687-698. doi:10.1016/S2213-2600(19)30198-5
- Epstein LJ, Kristo D, Strollo PJ Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263-276.
- Carvalho B, Hsia J, Capasso R. Surgical therapy of obstructive sleep apnea: a review. Neurotherapeutics. 2012;9(4):710-716. doi:10.1007/s13311-012-0141-x
- Haskell B, Voor M, Roberts A. A consideration of factors affecting palliative oral appliance effectiveness for obstructive sleep apnea: a scoping review, J Clin Sleep Med. 2021;17:833-848 https://doi.org/10.5664/jcsm.9018
- Sangalli L, YanezRegonesi F, Fernandez-Dial D, Moreno-Hay I. Selfreported improvement in obstructive sleep apnea symptoms compared to treatment response with mandibular advancement device therapy: a retrospective study. Sleep Breath. 2023;27(4):1577-1588. https://doi.org/10.1007/s11325-022-02754-4
- Haskell J, Haskell B, Spoon M, Feng C. The relationship of vertical skeletofacial morphology to oropharyngeal airway shape using cone beam computed tomography: Possible implications for airway restriction. Angle Orthod. 2014;84:548-554.
- Liu Y, Lowe AA, Fleetham JA, Park YC. Cephalometric and physiologic predictors of the efficacy of an adjustable oral appliance for treating obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 2001;120(6):639–647.
- Mostafiz W, Dalci O, Sutherland K, et al. Influence of oral and craniofacial dimensions on mandibular advancement splint treatment outcome in patients with obstructive sleep apnea. Chest. 2011;139(6):1331–1339.
- Glupker L, Kula K, Parka E, Babler W, Stewart K, Ghoneima A. Three-dimensional computed tomography analysis of airway volume changes between open and closed jaw positions. AJO-DO. 2015;147:426-434.
- Oatis C. Mechanics and pathomechanics of swallowing. In Oatis CA, ed. Kinesiology: The Mechanics and Pathomechanics of Human Movement. Baltimore, MD: Lippincott, Williams and Wilkins, 2009, pp 423–437.
- Rose EC, Barthlen GM, Staats R, Jonas IE. Therapeutic efficacy of an oral appliance in the treatment of obstructive sleep apnea: a 2-year follow-up. Am J Orthod Dentofacial Orthop. 2002;121(3):273–279.
- Posselt U. Movement areas of the mandible. J Prosthet Dent. 1957;7(3):375–385.
- Haskell J, McCrillis J, Haskell B, Scheetz J, Scarfe W, Farman A. Effects of mandibular advancement device (MAD) on airway dimensions assessed with cone beam computed tomography. Semin Orthod. 2009;15(2):132–158.
- Conley R, Cattaneo P, Haskell B. Characterization of the upper airway morphology and its changes in the apneic patient using cone beam computed. tomography. In: Kapila S, ed. Cone Beam Computed Tomography in Orthodontics Indications, Insights, and Innovations.1st ed. Oxford, UK: Wiley Blackwell, 2014, pp 273–291.
- Alves M Jr, Franzotti ES, Baratieri C, Nunes LK, Nojima LI, Ruellas AC. Evaluation of pharyngeal airway space amongst different skeletal patterns. Int J Oral Maxillofac Surg. 2012;41(7):814-819.
- Bilston L, Gandevia S. Biomechanical properties of the human upper airway and their effect on its behavior during breathing and in obstructive sleep apnea. J Appl Physiol 1985. 2014;116(3):314–324.
- Melo S, Li Z, Kamburoglu K, Enciso R, Scarfe W. Obstructive sleep apnea/ hypopnea syndrome. In: Scarfe WC, Angelopoulos C, eds. Maxillofacial Cone Beam Computed Tomography Principles, Techniques and Clinical Applications. 1st ed. Oxford, UK: Springer, 2018, pp 1–33.
- Sutherland K, Chan ASL, Ngiam J, Dalci O, Darendeliler MA, Cistulli PA. Awake multimodal phenotyping for prediction of oral appliance treatment outcome. J Clin Sleep Med. 2018;14(11):1879–1887.
- Jamieson A, Guilleminault C, Partinen M, Quera-Salva MA. Obstructive sleep apneic patients have craniomandibular abnormalities. Sleep. 1986;9(4):469-477.
- Marras W. Biomechanics of the human body. In: Salvendy G, ed. Handbook of Humans Factors and Ergonomics. 1st ed. New York, NY: John Wiley and Sons,1997, pp 233–267.
- Proffitt W. Contemporary Orthodontics. St. Louis, MO, Mosby, 2000, p 177.
- Hagen–Poiseuille equation Hagen–Poiseuille equation - Wikipedia Accessed 23 March 2022. https://en.wikipedia.org/wiki/Hagen%E2%80%93Poiseuille_equation
- Chung F, Subramanyam R, Liao P, Sasaki E, Shapiro C, Sun Y. High STOP-Bang score indicates a high probability of obstructive sleep apnoea. Br J Anaesth. 2012;108(5):768–775.
- Chervin R, Hedger K, Dillon JE, Pituch KJ. Pediatric Sleep Questionnaire (PSQ): Validity and reliability of scales for sleep disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1:21-32.
- Fernandez-Sanjuan P, Arrieta JJ, Sanabria J, et. al. Optimizing mandibular advancement maneuvers during sleep endoscopy with a titratable positioner: DISE-SAM Protocol. J Clin Med. 2022;11:658. https://doi.org 10.3390/jcm11030658
- Grauer D, Cevidanes LS, Styner MA, Ackerman JL, Proffit WR. Pharyngea airway volume and shape from cone-beam computed tomography: relationship to facial morphology. Am J Orthod Dentofacial Orthop. 2009;136(6):805–814.
- NejaimY, Aps JKM, Groppo FC, Haiter Neto F. Evaluation of pharyngeal spaceand its correlation with mandible and hyoid bone in patients with different skeletal classes and facial types. Am J Orthod Dentofacial Orthop. 2018;153(6):825–833.
- Shen HL, Wen YW, Chen NH, Liao YF. Craniofacial morphologic predictors of oral appliance outcomes in patients with obstructive sleep apnea. J Am Dent Assoc. 2012;143(11):1209–1217.
- Four Bar Linkage https://en.wikipedia.org/wiki/Four-bar_linkage Wikipedia Accessed 22 March 2022
- Taranto-Montemurro L, Sands S, Edwards B, et al. Desipramine improves upper airway collapsibility and reduces OSA severity in patients with minimal muscle compensation. Eur Respir J 2016;48(5):1340-1350.
- Grace KP, Hughes SW, Horner RL. Identification of the mechanism mediating genioglossus muscle suppression in REM sleep. Am J Respir Crit Care Med 2013;187(3):311-319.
- Gray H, Goss CM, eds. Anatomy of the Human Body, 28th Edition. Philadelphia, PA, Lea and Febiger, 1969, pp 169-172.
- Kevin O’Brian’s Orthodontic Blog. February 11, 2019. Part 2 of a brilliant summary of orthodontics and Obstructive Sleep Apnea. Accessed March 11, 2020 https://kevinobrienorthoblog.com/orthodontics-and-osa-part-2/.
SUBMISSION & CORRESPONDENCE INFORMATION
Submitted June 7, 2023
Submitted in final revised form November 17, 2023
Accepted for publication December 4, 2023
Address correspondence to: Dr. Bruce Haskell, DMD, PhD; Email: dr.bruce.haskell@gmail.com.
DISCLOSURE STATEMENT
The authors report no conflicts of interest.Appendix 1Cephalometric terminology |
Appendix 2ORAL APPLIANCE EFFICACY INDEX WORKSHEET: |
Appendix 3ORAL APPLIANCE EFFICACY INDEX WORKSHEET: |